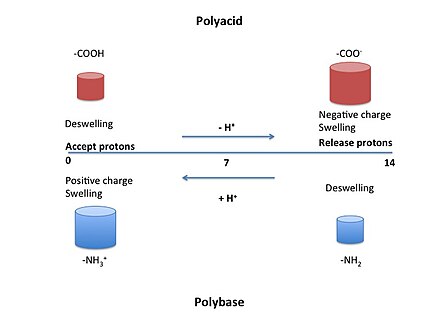
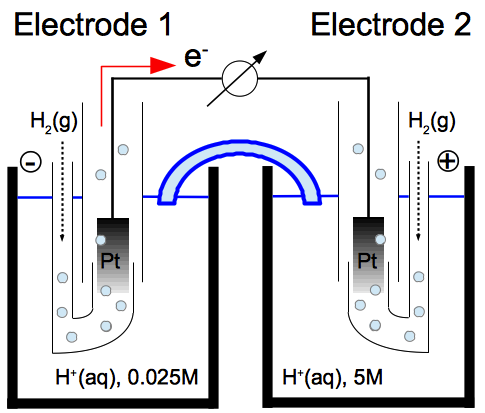
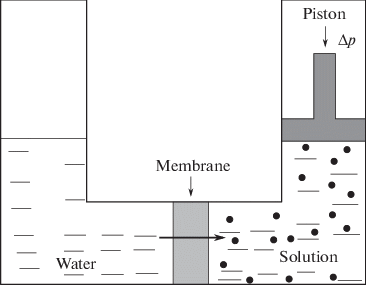
Countless macroscopic materials reversibly contract and then swell (or vice versa) as the concentration of a chemical agent increases and then decreases:

These materials can obviously do work. Here are two illustrations of how, by concentrating and deconcentrating the chemical agent (hydrogen ions), one can cyclically extract work from pH-sensitive macroscopic polymers able to lift weights:
Figure 4 here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1367611/pdf/biophysj00645-0017.pdf
Figure 16A here: https://pubs.acs.org/doi/pdf/10.1021/jp972167t.
Concentrating and then deconcentrating the chemical agent, per se, consumes no work if done quasistatically, e.g. with the help of a piston permeable to the solvent but impermeable to the chemical agent. In other words, the work lost in concentrating is compensated by the work gained in deconcentrating. Accordingly, in the case of pH-sensitive polymers, lifting the weight is the net work extracted from the cycle. The second law of thermodynamics is clearly violated.

These materials can obviously do work. Here are two illustrations of how, by concentrating and deconcentrating the chemical agent (hydrogen ions), one can cyclically extract work from pH-sensitive macroscopic polymers able to lift weights:
Figure 4 here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1367611/pdf/biophysj00645-0017.pdf
Figure 16A here: https://pubs.acs.org/doi/pdf/10.1021/jp972167t.
Concentrating and then deconcentrating the chemical agent, per se, consumes no work if done quasistatically, e.g. with the help of a piston permeable to the solvent but impermeable to the chemical agent. In other words, the work lost in concentrating is compensated by the work gained in deconcentrating. Accordingly, in the case of pH-sensitive polymers, lifting the weight is the net work extracted from the cycle. The second law of thermodynamics is clearly violated.