W
Wenderro
Guest
I saw that a lot of ppl are enthusiastic about a Polywell fusion reactor and now it will work maybe very soon so I tried to find more info about it. Unfortunately I couldn't find much so I'm left with gaps on understanding how it should work. Here it is what I understood about it so far:
One uses superconducting magnets positioned in a certain way to both contain the plasma and also accelerate electrons.
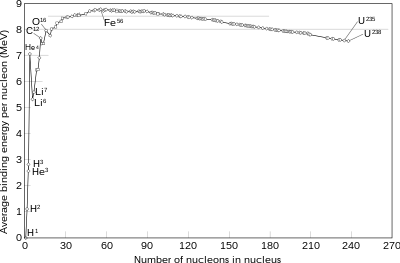
The basic idea is by accelerating electrons one overcomes the strong force making atoms fuse.
What I don't understand is:
Since one uses energy to produce fusion(overcome the strong force) how can the reactor break even? (since the energy released is exactly the strong force one overcame)
One uses superconducting magnets positioned in a certain way to both contain the plasma and also accelerate electrons.
The basic idea is by accelerating electrons one overcomes the strong force making atoms fuse.
What I don't understand is:
Since one uses energy to produce fusion(overcome the strong force) how can the reactor break even? (since the energy released is exactly the strong force one overcame)