Any chemical reactant can be concentrated and then deconcentrated quasistatically, e.g. by using a membrane permeable to the solvent but impermeable to the dissolved reactant:
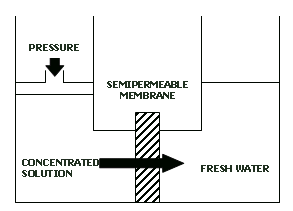
Obviously, the net work involved in the quasistatic cycle is zero (the work lost in concentrating is compensated by the work gained in deconcentrating).
Concentrating implies shifting the chemical equilibrium in one direction; deconcentrating implies shifting it in the other and closing the cycle. But shift-equilibrium cycles violate the second law of thermodynamics and thermodynamicists prohibit them:
"Suppose that, as indicated in the figure, the catalyst affects only the forward reaction. In its presence, the sum of the forward rates would clearly be larger than otherwise, while the backward rate would be unchanged. The position of equilibrium would therefore shift to the right, by the law of mass action. If we suppose further that the reaction produces heat q when it occurs, then a violation of the second law would be possible. We first allow equilibrium to be reached without the catalyst...and then add the catalyst, and heat δq is produced as the equilibrium is shifted. This heat is used to run a machine, and thus do work, cooling the system back to its original temperature in the process. We then remove the catalyst and the equilibrium shifts back. Heat δq is now extracted from the surroundings, which must warm the system back to the ambient temperature. A cycle has therefore been completed for which the net effect has been the isothermal conversion of heat energy into work, and a perpetual motion machine of the second kind has been found. We conclude that the supposed situation is impossible and that the catalyst must accelerate the forward and backward reactions equally." https://dtk.tankonyvtar.hu/bitstream/handle/123456789/8903/B9780120442621500128.pdf
When the shift-equilibrium cycle is triggered by a catalyst, the prohibition is easy: There is no such catalyst and that's it, thermodynamicists decree.
When the shift-equilibrium cycle is triggered by concentrating and subsequent deconcentrating of a chemical reactant, the prohibition is difficult, even impossible. Thermodynamicists will have to rely on the almost-two-century-old brainwashing of the scientific community.

Obviously, the net work involved in the quasistatic cycle is zero (the work lost in concentrating is compensated by the work gained in deconcentrating).
Concentrating implies shifting the chemical equilibrium in one direction; deconcentrating implies shifting it in the other and closing the cycle. But shift-equilibrium cycles violate the second law of thermodynamics and thermodynamicists prohibit them:
"Suppose that, as indicated in the figure, the catalyst affects only the forward reaction. In its presence, the sum of the forward rates would clearly be larger than otherwise, while the backward rate would be unchanged. The position of equilibrium would therefore shift to the right, by the law of mass action. If we suppose further that the reaction produces heat q when it occurs, then a violation of the second law would be possible. We first allow equilibrium to be reached without the catalyst...and then add the catalyst, and heat δq is produced as the equilibrium is shifted. This heat is used to run a machine, and thus do work, cooling the system back to its original temperature in the process. We then remove the catalyst and the equilibrium shifts back. Heat δq is now extracted from the surroundings, which must warm the system back to the ambient temperature. A cycle has therefore been completed for which the net effect has been the isothermal conversion of heat energy into work, and a perpetual motion machine of the second kind has been found. We conclude that the supposed situation is impossible and that the catalyst must accelerate the forward and backward reactions equally." https://dtk.tankonyvtar.hu/bitstream/handle/123456789/8903/B9780120442621500128.pdf
When the shift-equilibrium cycle is triggered by a catalyst, the prohibition is easy: There is no such catalyst and that's it, thermodynamicists decree.
When the shift-equilibrium cycle is triggered by concentrating and subsequent deconcentrating of a chemical reactant, the prohibition is difficult, even impossible. Thermodynamicists will have to rely on the almost-two-century-old brainwashing of the scientific community.




