A preposterous consequence of the second law of thermodynamics is that catalysts (enzymes in particular) accelerate the forward and backward reactions "equally", "by the same factor":
"Suppose that, as indicated in the figure, the catalyst affects only the forward reaction. In its presence, the sum of the forward rates would clearly be larger than otherwise, while the backward rate would be unchanged. The position of equilibrium would therefore shift to the right, by the law of mass action. If we suppose further that the reaction produces heat q when it occurs, then a violation of the second law would be possible. We first allow equilibrium to be reached without the catalyst...and then add the catalyst, and heat δq is produced as the equilibrium is shifted. This heat is used to run a machine, and thus do work, cooling the system back to its original temperature in the process. We then remove the catalyst and the equilibrium shifts back. Heat δq is now extracted from the surroundings, which must warm the system back to the ambient temperature. A cycle has therefore been completed for which the net effect has been the isothermal conversion of heat energy into work, and a perpetual motion machine of the second kind has been found. We conclude that the supposed situation is impossible and that the catalyst must accelerate the forward and backward reactions equally." https://dtk.tankonyvtar.hu/bitstream/handle/123456789/8903/B9780120442621500128.pdf
"It is important to recognize that when an enzyme (or any catalyst) lowers the activation energy for the reaction A → B, it also lowers the activation energy for the reaction B → A by exactly the same amount (see Figure 2-44). The forward and backward reactions will therefore be accelerated by the same factor by an enzyme..." https://www.ncbi.nlm.nih.gov/books/NBK26838/
Most enzymologists don't care about theoretical absurdities and intensively work on an effect called "catalytic bias", which is violation of the second law par excellence:
"In enzymology, there has been much interest in trying to elucidate what makes a particular enzyme faster in one direction (a property sometimes called the “catalytic bias”)." http://frenchbic.cnrs.fr/2022/09/22...catalysis-from-molecular-machines-to-enzymes/
"This has resulted in a deeper understanding of the hydrogenase model system and the ability to directly influence catalytic bias. Thus, the work presented here represents key progress towards developing unidirectional catalysts, and demonstrates the possibility of targeted, rational design and implementation of unidirectional catalysts." https://scholarworks.montana.edu/xmlui/handle/1/14621
"However, many enzymes reversibly convert their substrate and product, and if one is interested in catalysis in only one direction, it may be necessary to prevent the reverse reaction...This is the first demonstration, on a specific example, that slowing a step that is rate limiting only when the enzyme works in one direction is a general mechanism for biasing the enzyme in the other direction." https://hal.science/hal-01977597/document
"A research team from three U.S. Department of Energy (DOE) national laboratories and four universities found that subtle changes to the environment surrounding some enzymes can not only change the rate of a cellular reaction by a staggering six orders of magnitude but also its direction. That reversal—the root of the catalytic bias dilemma—is like speeding in one direction at 10 miles-per-second, then going in the opposite direction at 1,000,000 miles-per-second." https://www.pnnl.gov/news-media/remarkable-rate-return-catalytic-bias
Far from accelerating the forward and backward reactions "equally", "by the same factor", in this experiment
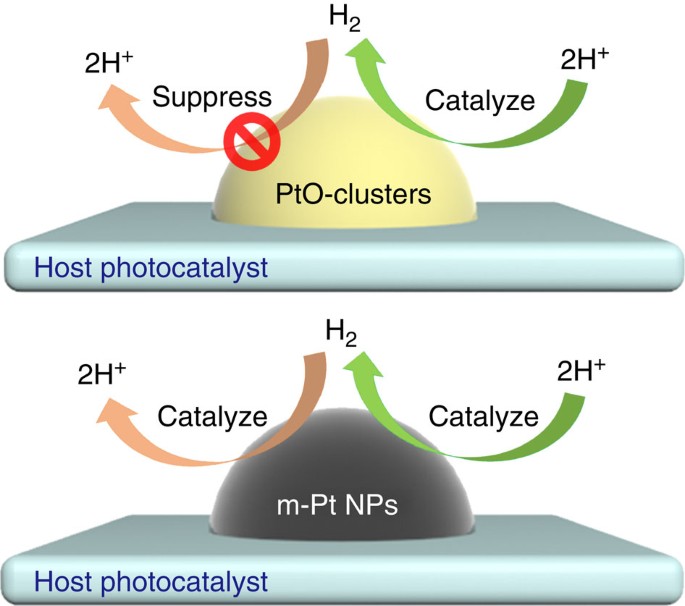
Yu Hang Li et al. Unidirectional suppression of hydrogen oxidation on oxidized platinum clusters https://www.nature.com/articles/ncomms3500
the catalyst, PtO, accelerates only the forward reaction, 2H+ → H_2, and SUPPRESSES H_2 → 2H+, the backward reaction:
"Suppose that, as indicated in the figure, the catalyst affects only the forward reaction. In its presence, the sum of the forward rates would clearly be larger than otherwise, while the backward rate would be unchanged. The position of equilibrium would therefore shift to the right, by the law of mass action. If we suppose further that the reaction produces heat q when it occurs, then a violation of the second law would be possible. We first allow equilibrium to be reached without the catalyst...and then add the catalyst, and heat δq is produced as the equilibrium is shifted. This heat is used to run a machine, and thus do work, cooling the system back to its original temperature in the process. We then remove the catalyst and the equilibrium shifts back. Heat δq is now extracted from the surroundings, which must warm the system back to the ambient temperature. A cycle has therefore been completed for which the net effect has been the isothermal conversion of heat energy into work, and a perpetual motion machine of the second kind has been found. We conclude that the supposed situation is impossible and that the catalyst must accelerate the forward and backward reactions equally." https://dtk.tankonyvtar.hu/bitstream/handle/123456789/8903/B9780120442621500128.pdf
"It is important to recognize that when an enzyme (or any catalyst) lowers the activation energy for the reaction A → B, it also lowers the activation energy for the reaction B → A by exactly the same amount (see Figure 2-44). The forward and backward reactions will therefore be accelerated by the same factor by an enzyme..." https://www.ncbi.nlm.nih.gov/books/NBK26838/
Most enzymologists don't care about theoretical absurdities and intensively work on an effect called "catalytic bias", which is violation of the second law par excellence:
"In enzymology, there has been much interest in trying to elucidate what makes a particular enzyme faster in one direction (a property sometimes called the “catalytic bias”)." http://frenchbic.cnrs.fr/2022/09/22...catalysis-from-molecular-machines-to-enzymes/
"This has resulted in a deeper understanding of the hydrogenase model system and the ability to directly influence catalytic bias. Thus, the work presented here represents key progress towards developing unidirectional catalysts, and demonstrates the possibility of targeted, rational design and implementation of unidirectional catalysts." https://scholarworks.montana.edu/xmlui/handle/1/14621
"However, many enzymes reversibly convert their substrate and product, and if one is interested in catalysis in only one direction, it may be necessary to prevent the reverse reaction...This is the first demonstration, on a specific example, that slowing a step that is rate limiting only when the enzyme works in one direction is a general mechanism for biasing the enzyme in the other direction." https://hal.science/hal-01977597/document
"A research team from three U.S. Department of Energy (DOE) national laboratories and four universities found that subtle changes to the environment surrounding some enzymes can not only change the rate of a cellular reaction by a staggering six orders of magnitude but also its direction. That reversal—the root of the catalytic bias dilemma—is like speeding in one direction at 10 miles-per-second, then going in the opposite direction at 1,000,000 miles-per-second." https://www.pnnl.gov/news-media/remarkable-rate-return-catalytic-bias
Far from accelerating the forward and backward reactions "equally", "by the same factor", in this experiment
Yu Hang Li et al. Unidirectional suppression of hydrogen oxidation on oxidized platinum clusters https://www.nature.com/articles/ncomms3500
the catalyst, PtO, accelerates only the forward reaction, 2H+ → H_2, and SUPPRESSES H_2 → 2H+, the backward reaction:



