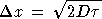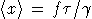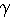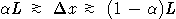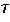Nature: "Second law broken. Researchers have shown for the first time that, on the level of thousands of atoms and molecules, fleeting energy increases violate the second law of thermodynamics. [...] They found that over periods of time less than two seconds, variations in the random thermal motion of water molecules occasionally gave individual beads a kick. This increased the beads' kinetic energy by a small but significant amount, in apparent violation of the second law." http://www.nature.com/news/2002/020722/full/news020722-2.html
This was published in 2002 and immediately forgotten. In thermodynamics, crimestop is even stronger than in relativity:
George Orwell: "Crimestop means the faculty of stopping short, as though by instinct, at the threshold of any dangerous thought. It includes the power of not grasping analogies, of failing to perceive logical errors, of misunderstanding the simplest arguments if they are inimical to Ingsoc, and of being bored or repelled by any train of thought which is capable of leading in a heretical direction. Crimestop, in short, means protective stupidity."
Still I am going to show in this thread that violations of the second law of thermodynamics are, actually, commonplace.
"However, in experiments in which a capacitor is submerged in a dielectric liquid [e.g. deionized water] the force per unit area exerted by one plate on another is observed to decrease. [...] This apparent paradox can be explained by taking into account the DIFFERENCE IN LIQUID PRESSURE in the field filled space between the plates and the field free region outside the capacitor." http://farside.ph.utexas.edu/teaching/jk1/lectures/node46.html
The submerged capacitor experiment is able to violate the second law of thermodynamics, and the DIFFERENCE IN LIQUID PRESSURE is essential. If a small hole is punched in one of the plates, the high interplate pressure will produce a permanent flow through the hole. This flow can be harnessed to do work, at the expense of ambient heat.
Water in an electric field automatically becomes a perpetual-motion machine of the second kind. Vigorous motion is generated that can do work (e.g. by rotating waterwheels) at the expense of ambient heat (there is no other source of usable energy):
View: https://www.youtube.com/watch?v=17UD1goTFhQ
This was published in 2002 and immediately forgotten. In thermodynamics, crimestop is even stronger than in relativity:
George Orwell: "Crimestop means the faculty of stopping short, as though by instinct, at the threshold of any dangerous thought. It includes the power of not grasping analogies, of failing to perceive logical errors, of misunderstanding the simplest arguments if they are inimical to Ingsoc, and of being bored or repelled by any train of thought which is capable of leading in a heretical direction. Crimestop, in short, means protective stupidity."
Still I am going to show in this thread that violations of the second law of thermodynamics are, actually, commonplace.
"However, in experiments in which a capacitor is submerged in a dielectric liquid [e.g. deionized water] the force per unit area exerted by one plate on another is observed to decrease. [...] This apparent paradox can be explained by taking into account the DIFFERENCE IN LIQUID PRESSURE in the field filled space between the plates and the field free region outside the capacitor." http://farside.ph.utexas.edu/teaching/jk1/lectures/node46.html
The submerged capacitor experiment is able to violate the second law of thermodynamics, and the DIFFERENCE IN LIQUID PRESSURE is essential. If a small hole is punched in one of the plates, the high interplate pressure will produce a permanent flow through the hole. This flow can be harnessed to do work, at the expense of ambient heat.
Water in an electric field automatically becomes a perpetual-motion machine of the second kind. Vigorous motion is generated that can do work (e.g. by rotating waterwheels) at the expense of ambient heat (there is no other source of usable energy):