Re: Simulations Show Liquid Water Could Exist on Mars / New Phoe
I especially thought of rlb2 and Andrew when I saw this report:
(I still want to point out that the liquid droplets observed by Phoenix could've been the liquid water-hydrazine eutectic also. For some reason, this possibility still hasn't been considered in the reports on the Phoenix doplets. See my post on the other thread on this subject.)
http://news.bbc.co.uk/2/hi/science/nature/7958471.stm
Briny pools 'may exist on Mars'
By Paul Rincon
Science reporter, BBC News, The Woodlands, Texas
The probe had surpassed its expected lifetime by more than two months
Pools of salty water might be able to exist just below the surface of Mars, planetary scientists believe.
Researchers previously thought water existed largely as ice or as vapour on Mars, because of the low temperatures and atmospheric pressure.
But Nasa's Phoenix lander has shown the presence in Martian soil of perchlorate salts, which can keep water liquid at temperatures of minus 70C.
Pockets of brine might form when soil interacted with ice.
Researchers have been discussing the idea at the 40th Lunar and Planetary Science Conference (LPSC), here in The Woodlands, Texas.
They were presenting some of the first scientific results from Phoenix, which touched down on Mars's northern plains on 25 May 2008.
"I do think those pools might exist. But there's still more to know about the properties of these perchlorate solutions, such as what their vapour pressure is," Dr Mike Hecht, from Nasa's Jet Propulsion Laboratory (JPL) in Pasadena, California, explained.
Soil dampness
Phoenix used thrusters to slow its descent to the surface. And these blew away topsoil, exposing water-ice just centimetres beneath.
Dr Hecht said: "Here are all these perchlorate salts right under them, by a few centimetres, is a slab of [water ice]. It doesn't take much of a stretch of the imagination to say that those two materials will interact.
One of Phoenix's great achievements was to "touch" the water-ice
"And once you get dampness, the perchlorate is very soluble and it will become mobile."
On Earth, perchlorates - salts derived from perchloric acid - are used in solid rocket fuel, fireworks and airbags. Scientists are just starting to understand the important roles they may play on Mars.
Dr Hecht said that forming pockets of liquid on Mars would require just the right concentrations of perchlorate salts. He commented: "In this case we have very little perchlorate and vast slabs of ice, so I can imagine we have an excess of water. This means you would form a pool of low temperature brine if the two ever interacted."
Other researchers cautioned that the concentrations of these salts found at the Phoenix landing site remained a small component of the overall soil chemistry, and that more had to be done to test the idea.
Nevertheless, Dr Hecht said the discovery of these compounds made the Red Planet seem more Earth-like in several respects.
Big tilt
Perchlorates might be controlling the amount of water vapour in the midday atmosphere, according to separate evidence presented by Dr Troy Hudson of JPL.
And their presence might also explain why neither Phoenix nor the 1970s Viking landers found any firm evidence for "organics" - molecular compounds which contain carbon (though excluding carbonates for historic reasons).
These molecules are a crucial component in the search for possible biology on the Red Planet.
"The perchlorates, as you heat them in the oven (onboard Phoenix), release their oxygen and combust the organics," Peter Smith, the mission's chief scientist, told the conference.
"It's ironic: the two compete as you heat them. We did see CO2 release, but we're not sure whether that was from organics or not."
Professor Smith said several lines of evidence pointed to the past action of liquid water on the northern plains. These included the presence of aqueous minerals, cloddy, cemented soil and the discovery that some of the ice was "segregated", as if it had melted.
"It's probable that in a warmer, wetter climate, as when the obliquity (the extent to which Mars is tilted on its axis) changes, this could be a place where liquid water is found. That doesn't mean it's a lake. It just means that the soil is wet," Professor Smith, from the University of Arizona, explained.
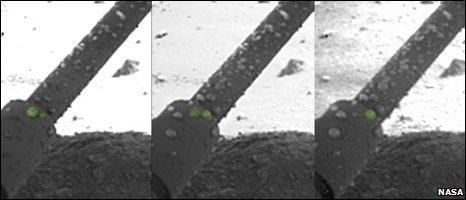
Dr Nilton Renno thinks he has seen evidence of salty liquid-water droplets
The discovery of calcium carbonate in the soil is also suggestive of the past action of liquid water. The substance is found in rocks all over Earth and is the main component in limescale.
Peter Smith said it occurred at levels of 3-5% at the Phoenix landing site, probably forming as carbon dioxide from the Martian atmosphere dissolved into liquid water, forming a weak acid which leached calcium out of the soil.
But Dr Nilton Renno, from the University of Michigan, US, presented evidence that droplets of liquid water could actually be seen in photographs of a strut of the spacecraft's landing leg.
"The (spheroids) move, drip and merge," Dr Renno explained.
But Mike Hecht and Dr Tom Pike, from Imperial College London, UK, believe the droplets are more likely to be frost.
"The photographs are clipped from the corners of relatively low resolution images, so the number of pixels across those droplets is very small. Trying to ascribe shapes to them, to say they are spheres - which are characteristic of liquid - is going beyond the quality of the images," said Mike Hecht.
Secondly, he thought the thermodynamics of the Martian environment were not consistent with the relatively large changes in the sizes of droplets seen in the images.
Eventual demise
Dr Renno told the conference that ice particles were usually not just spheroidal, and did not move in the way the droplets did.
Mike Hecht said frost was able to move more readily in the Martian environment than it did on Earth because of the thin air.
However, the JPL scientist emphasised his agreement with Dr Renno on most areas concerning the properties of perchlorates at the Phoenix landing site.
Launched from Earth in August 2007, Phoenix landed further north than any previous mission to the Martian surface.
It conducted science operations for more than five months before succumbing to the cold and dark of the Martian winter. The robot dug, scooped, baked, sniffed and tasted the Martian soil to test whether it has ever been capable of supporting life.
It became the first mission to Mars to sample the water-ice it found just centimetres below the topsoil. Chunks of ice were seen to vaporise before the lander's cameras.